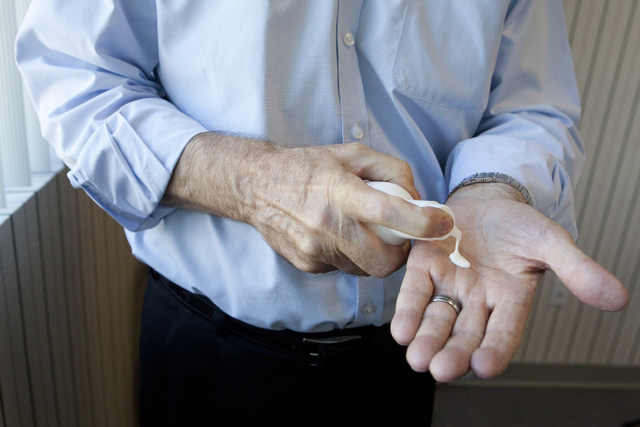
Canadian Olympic team using sanitizing lotion made by Las Vegas company
Las Vegans watching the summer Olympics may be surprised to learn that there’s a local connection to the Canadian national team.
Skinvisible Pharmaceuticals Inc., a local research development company, created a nonalcohol sanitizing lotion that is being used by the Canadian Olympic team.
“This, to us, is a huge moment,” said Terry Howlett, president and CEO of the company.
The product, DermSafe, has not yet been approved by the U.S. Federal Drug Administration. But it has been approved in Canada and is being produced through the company’s subsidiary Kintari Canada Inc. in Toronto.
Howlett moved to Las Vegas from Vancouver, Canada, in the late 1990s to run the pharmaceutical company. After selling his software development business in Canada, Howlett said, he bought an existing local company and associated products and opened the lab in southeast Las Vegas.
Howlett said his company is the first to develop the nonalcohol hand sanitizer, DermSafe. The five-employee company works on other skin-related products and has 14 patents, he said.
Once applied, DermSafe stays on for more than four hours, and any bacteria or viruses that touch the skin are killed on contact, the company said. Howlett said he contacted the Canadian sailing team in December after reading about contamination in the water around the Olympic host city Rio de Janeiro. In water quality tests carried out by The Associated Press ahead of the games, an investigation found levels of contamination that it reported could pose health risks.
The company has donated 1,000 bottles of the product, $15,400 U.S. dollars worth, to the Canadian Olympic team.
“The product has proven to be exceptional under the rigors of training in Rio, to date both athletes and coaches have felt confident and protected, while having no side effects for either product use or the constant exposure to Rio’s water quality,” said Ken Dool, high-performance director at Sail Canada, in a statement.
Howlett estimates that getting FDA approval could take a few years and millions of dollars in studies.
According to FDA spokeswoman Andrea Fischer, the product’s use of one of the key ingredients, chlorhexidine gluconate, is not covered by certain standards.
“Although there are health care antiseptic drugs containing chlorhexidine gluconate that are approved under a New Drug Application (NDA), chlorhexidine gluconate has not been demonstrated to be eligible for review under the OTC drug monograph process for use in consumer antiseptic rub products,” said Fischer, in an email.
Correction: A previous version of this story incorrectly identified Terry Howlett’s city of origin.
Contact Alexander S. Corey at acorey@reviewjournal.com or 702-383-0270. Find @acoreynews on Twitter.