FDA adviser: COVID-19 vaccine could provide ‘a way out of this mess’
In what a top U.S. vaccine expert called a “scientific tour de force,” the first doses of a vaccine to counter the scourge of COVID-19 could be shipped to Las Vegas and communities across the U.S. as soon as this week.
Development and testing of a vaccine, a process that normally takes a decade or more, has been condensed into 11 months, propelled by the laser focus of scientists along with the infusion of billions of federal dollars.
Two early candidates for approval that use a new gene-based technology are far exceeding expectations in large clinical trials conducted in Las Vegas and hundreds of other cities on tens of thousands of participants. Developers say their vaccines are more than 94 percent effective and have produced minimal side effects.
If you’d asked 1,000 scientists in the U.S. 11 months ago if it would be possible to reach this point so fast, what might their response have been?
“I think you would’ve not had one scientist that would’ve raised their hand and said that they thought that was possible. Not one,” Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told the Review-Journal.
Offit is also a member of the independent advisory committee for the Food and Drug Administration that will soon recommend whether the federal agency should authorize the emergency use of the two vaccines.
On Thursday, the committee will review evidence and recommend whether the agency should authorize emergency use of what would be the first vaccine in the U.S., a drug developed by American pharmaceutical company Pfizer and German firm BioNTech. The panel will meet again on Dec. 17 to consider a second prospective vaccine, this one developed by U.S. biotech company Moderna Inc. in conjunction with the National Institutes of Health.
At each meeting, the panel will consider questions on the minds of many Americans about the safety and effectiveness of a prospective vaccine against a disease that this year has killed more than 2,300 people in Nevada, 280,000 in the U.S. and 1.5 million around the world.
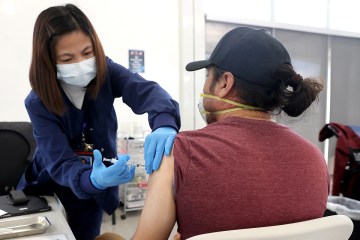
The first lots of the vaccines, which are delivered by injection and require two doses, already have been manufactured in anticipation of affirmative decisions by the FDA, allowing the nearly immediate shipment of allocations to communities.
A new technology
The Pfizer and Moderna vaccines rely on a technology called messenger RNA, or mRNA, a reference to the molecules that carry DNA instructions for making proteins in the human body.
They work very differently from their predecessor vaccines.
“To trigger an immune response, many vaccines put a weakened or inactivated germ into our bodies,” the Centers for Disease Control and Prevention explains to illustrate how the new approach differs from the old. “Not mRNA vaccines. Instead, they teach our cells how to make a protein — or even just a piece of a protein — that triggers an immune response inside our bodies.
“That immune response, which produces antibodies, is what protects us from getting infected if the real virus enters our bodies.”
The mRNA vaccines for COVID-19 give instructions to cells to make a harmless piece of the “spike” protein on the surface of the virus.
“Our immune systems recognize that the protein doesn’t belong there and begin building an immune response and making antibodies, like what happens in natural infection against COVID-19,” the CDC states on its website.
There are currently no licensed mRNA vaccines in the United States, though researchers have been studying the technology for decades.
An advantage of the mRNA vaccines is that they can be manufactured more rapidly than traditional vaccines, which have to be grown in a lab. With the mRNA vaccine, the body itself grows the protein to stimulate the immune system response.
This advantage of speed helps to explain why the first two COVID-19 vaccines on the horizon are mRNA vaccines.
Safety questions
The velocity at which vaccines have been developed raises questions about whether corners have been cut in terms of safety.
“I think the real answer is no,” Offit said. “We just speeded up the process. We speeded up the process because the federal government was willing to take the risk out of it for pharmaceutical companies. The federal government said, ‘Let’s basically put $24 billion into this.’”
The drug companies are seeking to distribute the vaccine under FDA emergency use authorization, which allows shots to be given to certain people while studies of safety and effectiveness are ongoing.
Should emergency use be authorized, Nevada officials have said that the first people in the state to receive the vaccine will be medical personnel at hospitals, followed by staffers and residents of long-term care facilities and critical infrastructure personnel, such as a law enforcement and Department of Corrections workers.
Full FDA approval of a vaccine, which would allow vaccinations of the general population, will likely require six months of safety follow-up as well as extensive inspections of company manufacturing sites. The leading vaccine-makers are not expected to complete that process until spring or summer.
Before considering authorizing emergency use, the FDA has required the vaccine companies to provide two months of safety data dating to the time that trial participants received their second doses.
This requirement is designed to ensure that “you don’t have a serious adverse event that is relatively uncommon,” Offit said. However, as with any medical product, longer study will be required to determine whether there is the possibility of a rare, serious side effect.
Nevada Gov. Steve Sisolak and other state officials have said that no vaccine will be given in Nevada until it’s found to be safe.
During a virtual press conference Tuesday, Sisolak reiterated that Nevada had joined other Western states to form a working group to independently review the safety and efficacy of any vaccine approved by the FDA for distribution.
The goal is to provide “an additional layer of confidence when it comes to COVID-19,” Sisolak said. “This verification process is happening in lockstep with the federal approval agency process.”
He said he did not expect the additional step to delay delivery of a vaccine to residents.
‘More hurdles’ in process
However, Offit thinks that the decision by some states to form their own review committees “creates more hurdles than are necessary” and reflects distrust in the FDA.
He acknowledged that the FDA’s controversial authorization of emergency use for hydroxychloroquine, a drug touted by President Donald Trump, for treatment of COVID-19 earlier this year might have hurt the agency’s credibility.
“It was clear at that point that it seemed that the administration was influencing the FDA’s decision,” he said. “But that’s changed now. I really do think that.”
He believes the FDA approval process is sound and vowed that the agency and its independent advisory committee will carefully review the vaccine data.
“We’ll make a recommendation based on whether we think we would take these vaccines ourselves or give them to our family before we recommend them for other people’s families,” said Offit, co-inventor of the rotavirus vaccine recommended for infants by the Centers for Disease Control and Prevention.
“If you have states that are giving the vaccine and states that aren’t giving the vaccine, or states that are giving the vaccine to one group but not another, and then other states that are giving it to different groups, I just think it’s chaotic and it’s unnecessary,” he said. “I understand the sentiment, but I just think it no longer applies.”
Despite the favorable early signs for the new vaccines, Offit sounded a note of caution when he spoke with reporters during a recent briefing.
“The only thing we’ve seen so far are press releases,” he said. “I really would like to have seen either a rapid publication (of data from the clinical trials) in a science or medical journal so you could really drill down on those details. When we say it’s (nearly) 95 percent effective, for whom and for how long?”
Still, he sees the reason for great optimism regarding a vaccine.
“I think what we’re seeing is remarkable. It is a tour de force. It is a scientific tour de force,” he said.
“But like all of us … you’re just a little nervous, because the virus is new and because these strategies are new, to go too far overboard,” he continued. “But I think you should be incredibly optimistic that we now have in hand, in all likelihood, a way out of this mess.”
Dr. Paul Offit’s comments were made during a question-and-answer session in late November with National Press Foundation vaccine fellows.
The Associated Press contributed to the report.
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.