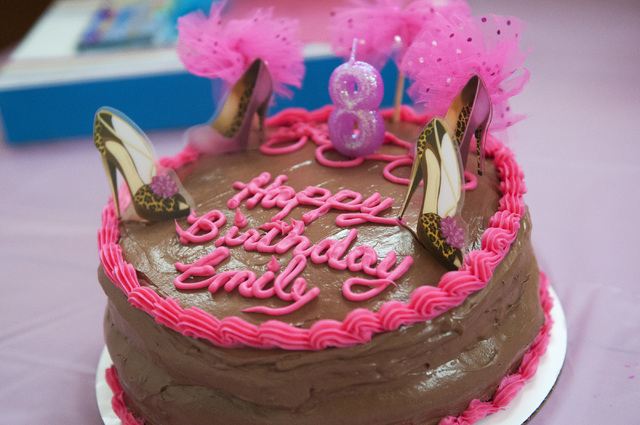Parents lobby for, and get, drug to help daughter manage form of hemophilia
At the party celebrating her eighth birthday, Emily Bartko and a classmate from St. Elizabeth Ann Seton Catholic School, Sidney Meriwether, danced along with performers on the big-screen TV.
Their concern was only about whether they were in step.
“This is so cool,” Emily told Sidney as they moved in tandem.
Nearby, Emily’s big sister, 10-year-old Abbey, held their little brother, 4-month-old A.J.
“You’re so cute,” Abbey said as she nuzzled the baby boy.
That snapshot of what played out before a smiling John and Alison Bartko the other day in their northwest Las Vegas home — grandparents and a godmother were also on hand — is seemingly untroubled Americana at its best, where good times are the norm, the kind of scene Norman Rockwell would have painted.
But the Bartkos, both are pharmacists and the parents of Abbey, Emily and A.J., are the first to tell you that a snapshot is only that, it can’t capture the worry and grief and tenacity and medical acumen that accompanied their family to this place in time, where a rare, incurable bleeding disorder can at least be managed. However, the condition still poses a very real threat to shorten the lives of two of their three children.
“Emily could have bled to death from her umbilical cord (which had been clamped after birth when it came loose),” said Dr. Jonathan Bernstein, the Las Vegas physician who discovered that the child born Sept. 9, 2005, suffered with a rare form of hemophilia in which she is missing Factor 1, a protein known as fibrinogen that helps blood to clot.
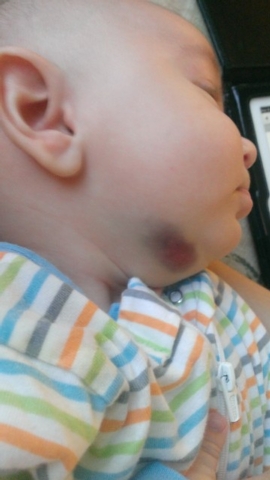
The Bartkos said A.J. also bled from the umbilical cord area after his birth, but his bleeding was easier to control. Because he is so young and largely immobile, rendering him less likely to injure himself and bleed, he has yet to start the medication that Emily must be infused with every two weeks.
With the Nevada chapter of the National Hemophilia Foundation holding its fifth annual Nevada Walk/5K for Hemophilia on Saturday at Floyd Lamb at Tule Springs Park, the Bartkos decided to go public with their family’s struggle in the hopes of raising awareness of a disease that the federal Centers for Disease Control and Prevention estimates affects some 20,000 people in the United States. The event will benefit those living with bleeding disorders and their families.
“We need a cure,” Alison Bartko said, stressing that a public perception of the disease — that it affects only boys — is wrong.
“The majority of cases are boys, but girls are affected, too … some milder cases are never even diagnosed, but people who seem to bleed quite a bit, say with nosebleeds, should be checked out.”
Without a diagnosis, a person with a mild case of hemophilia who uses no prophylactic treatment is likelier to be subject to hemorrhages in joints, creating potentially disabling arthritis.
Bartko also said unseen internal bleeds, cerebral hemorrhages in particular, carry the most risk, especially for kids. That’s why so many kids with the disease wear helmets, including Emily until very recently. Bartko said her daughter is now mature enough to be careful about head bumps.
Hemophilia, an inherited bleeding disorder in which the blood does not clot properly, can lead to spontaneous bleeding as well as bleeding and pronounced bruising following injuries or surgeries. With Emily’s Factor 1 deficiency, just crawling or walking would leave her legs or the bottoms of her feet black and blue. Putting her in a car seat would leave bruises over much of her body.
The Bartkos dressed her in a variety of pads and headgear in an attempt to protect her.
Fortunately, the efforts of Bernstein and the Bartkos to acquire a medication used only in Europe has given Emily a new lease on life, one largely free from bruising and bleeding. Still, there is always the risk of her developing a tolerance for the medication, or of incurring a serious injury where internal bleeding can’t be stopped.
Until Emily’s birth, the Bartkos had no idea that both of them carried a recessive gene for Factor 1 hemophilia, a disorder that affects only one or two people in a million. It is inherited in an autosomal recessive fashion, which means it affects men and women equally. Bernstein said there are between 250 to 300 Americans living with the disease.
Abbey was born without the disorder.
“That’s why we were so amazed when Dr. Bernstein told us what Emily had,” John Bartko said. “So far we haven’t been able to find anybody on either side of our family who had hemophilia. And doctors don’t know why Abbey doesn’t have it.”
Abbey and Emily were both conceived after the Bartkos went through fertility treatment. When A.J. came along, it was a shock to both parents.
“We were told it was highly unlikely we could have children naturally,” John Bartko said. “When Alison said she was pregnant, you could have knocked me over with a stiff breeze.”
As observant Catholics, the Barkos never considered abortion. Given that a drug has now given Emily a much more natural childhood, both parents hope A.J. can escape the challenges his sister initially faced.
Treatments for some forms of the disease, including the RiaSTAP for Factor 1 deficiency that Emily now uses every two weeks, are more available and much safer than the treatments of 40 years ago.
From the late 1970s to the mid-1980s, the National Hemophilia Foundation estimates that half of all people with the disease became infected with HIV through blood products. Because of new viral killing methods, which include heat treatment and solvent-detergent cleansing, however, none of the main blood products used in treatment has transmitted HIV since 1986.
As of 1997, there also have been no reports of hepatitis C transmission through clotting factor blood products that have been subject to new treatment methods, according to the hemophilia foundation.
Yet the Bartkos had some anxious moments after Bernstein had to give Emily two doses of the blood product cryoprecipitate to stop her umbilical cord bleeding at Sunrise Hospital.
“We received a letter from the CDC saying it could have been tainted with HIV and hepatitis,” John Bartko said. “We’ve had her tested every six months and she’s tested negative every time but it did cause us some real anxiety.”
After his Factor 1 hemophilia diagnosis of Emily, Bernstein told her parents something that Alison Bartko says she’ll always remember — “There is no treatment available right now.”
She wondered whether her daughter, who even suffered handprint bruises from being picked up by her and her husband, would die. Or whether her life would have to be spent away from school and other activities children enjoy.
As the Bartkos worked to keep their daughter safe from injury and prayed for a treatment that would work, Bernstein learned there was going to be a study of a drug in the United States that had long been used in Europe for Factor I deficiency. It turned out, however, that Emily was too young to participate.
Bernstein didn’t take no for an answer. He petitioned the Food and Drug Administration for compassionate use of the drug. Seven months later, on Oct. 2, 2007, Emily received her first infusion, one that nurse practitioner Amber Federizo now gives her every 14 days through an IV for about a half-hour at the Hemophilia Treatment Center near Sunrise Hospital.
Immediately after the first infusion, Alison Bartko said, she and her husband noticed a change in their little girl. She was happier, more content. Her bruising diminished and she no longer required pain medication. She wanted to dance. Smiles replaced grimaces.
In 2009, the Bartkos and Bernstein went before the FDA in Washington, D.C., to argue that RiaSTAP should be readily available to all Americans that present with the disease, not just to those who petition for use.
“Having access to a fibrinogen concentrate has been nothing less than life-changing for Emily and our family,” Alison Bartko said in her address to an FDA panel. “It seems almost crazy to have to justify a medication that would help our child. … The ability to heal is not an option for Emily. We are asking you to help us and the other children in the United States who suffer from this deficiency.”
About a year later, the FDA approved use of the drug in the United States. Unfortunately, Bernstein said, those without insurance can have a difficult time getting it. Clotting drugs, he said, sometimes cost around $1 million a year.
Until there is a cure, Emily and later A.J. will probably use RiaSTAP, Bernstein said.
He said he must always fine-tune the prophylactic dosage, because if he would give too much to either of the children they could experience dangerous blood clots.
“It’s wonderful to see her happy,” Bernstein said.
“This drug is giving Emily a far more normal childhood and I’m sure it will with A.J., too,” John Bartko said. “We’re hoping that stem cell research will help come up with a cure. That’s why there will be walks all over the country on Sept. 21. So we can raise the money for the research we need.”
Contact reporter Paul Harasim at pharasim@reviewjournal.com or 702-387-2908.
TAKE A WALK
The Nevada Chapter of the National Hemophilia Foundation will sponsor its fifth annual Nevada Walk/5K for Hemophilia, which benefits those suffering from bleeding disorders in the Silver State. It will take place Saturday at Floyd Lamb Park at Tule Springs, with check-in beginning at 7:30 a.m. Sign up for the walk online by visiting www.hemophilia.org/walk and click on NV. Call 702-564-4368 for more details.