Las Vegas man is global trailblazer for new lymphoma treatment
A Las Vegas man is the first patient in the world to participate in a clinical trial of a treatment for a rare form of non-Hodgkin’s lymphoma.
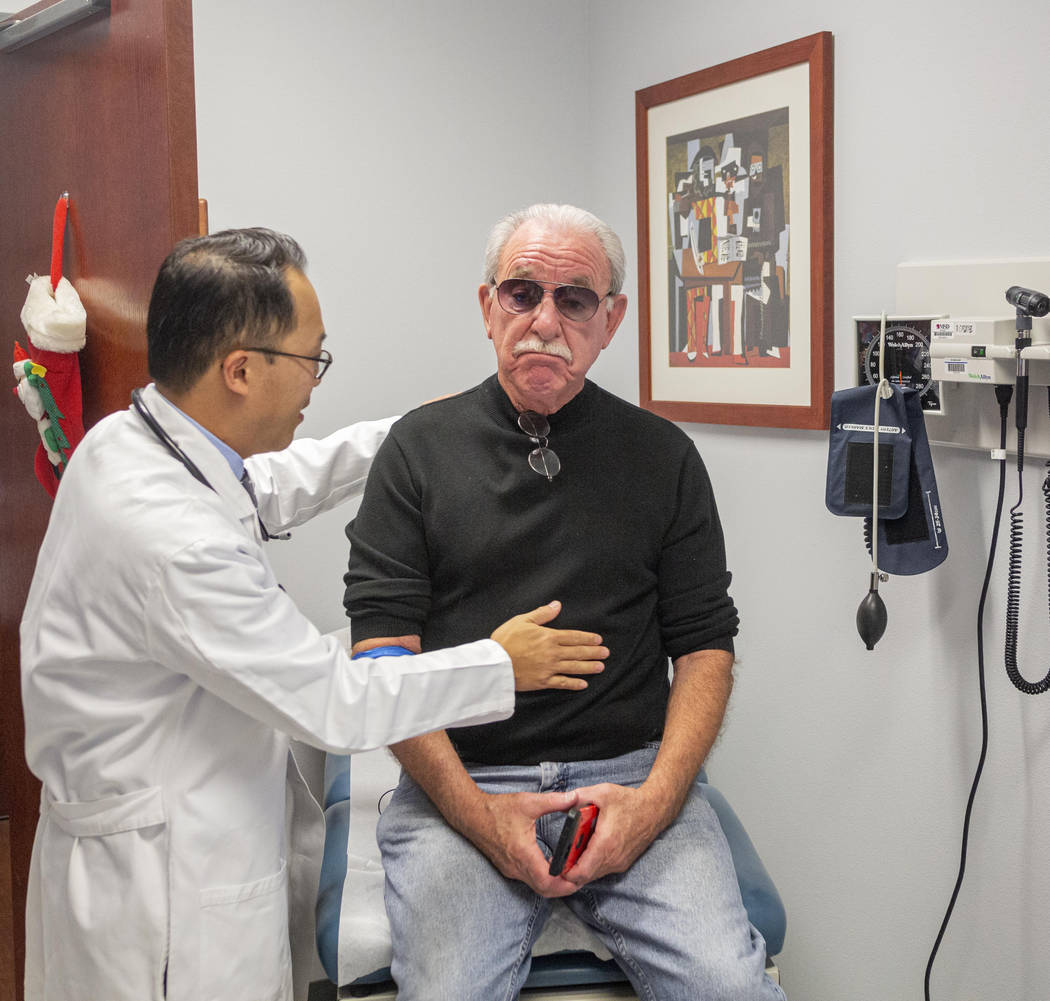
Fred Warnick, 73, began the drug trial in August at Comprehensive Cancer Centers of Nevada. The results so far have been promising.
“His lymph nodes are nearly shrunken down to normal,” said Dr. Anthony Nguyen, Warnick’s oncologist. “He’s in near complete remission.”
Warnick was diagnosed in June with advanced stage 4 mantle cell lymphoma, a cancer of the lymphocytes, a specific type of white blood cell. Lymphocytes are found in the lymph nodes, pea-sized glands in places such as the neck, groin and armpits.
Traditional treatment can include “hard chemotherapy” delivered in a hospital and a bone marrow transplant requiring hospitalization for a month or longer, Nguyen said.
Instead, Warnick takes four pills a day of zanubrutnib to inhibit the growth of cancer cells. Every 28 days, he receives intravenously a drug called rituximab, which helps the immune system destroy cancer cells.
In an appointment at a Comprehensive Cancer clinic on Wednesday, Warnick told Nguyen he was experiencing some insomnia but hadn’t lost his hair or appetite.
“I feel fine. I’m starting to get depressed I feel so good,” Warnick, a father of three, step-father to three and grandfather to 10, said jokingly to a reporter.
In June, Warnick went to an urgent care center complaining of bloating and pain in his abdomen and legs. He later went to Comprehensive Cancer Centers, where the doctors found that he had cancer in his chest, abdomen, pelvis and “hiding in the kidneys,” Nguyen said.
Since treatment began in August, scans show not only that Warnick’s lymph nodes have shrunken to a normal size but that tumors in his armpit had disappeared.
The U.S. Food and Drug Administration last month approved the use of zanubrutnib for adult patients who already have undergone one form of treatment.
The current drug trial focuses on pairing zanubrutnib and rituximab as the first line of treatment for patients over age 70, who may be more frail and less able to withstand other treatments, Nguyen said.
The trial is to be conducted at 206 sites across the globe, including 21 in the U.S. Comprehensive Cancer Centers was the first of the locations to activate the trial.
Comprehensive Cancer Centers is currently conducting 170 drug trials, said Nguyen, and the FDA has approved 86 drugs from its previous trials.
An increase in clinical trials locally means that fewer Southern Nevadans need to go out-of-town for life-extending treatment, Nguyen said. Trial participants also receive costly drugs free of charge.
Clinical trials “give us more options for our patients,” he said, “and more hope.”
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.