Complaint led state to halt Las Vegas clinic’s COVID-19, antibody tests
The Nevada Department of Health and Human Services confirmed Wednesday that it had issued a cease-and-desist order to a Las Vegas clinic conducting rapid blood tests for COVID-19.
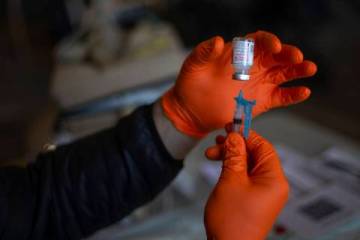
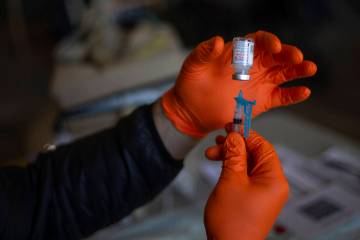
As reported Tuesday by the Review-Journal, the department halted operations at Sahara West Urgent Care and Wellness, which last week began using the rapid test to screen for COVID-19 as well as for the antibodies developed after a person has been ill with the new coronavirus.
Since early in the outbreak, the clinic had been conducting drive-through testing that relied upon the use of a nasal swab, with the laboratory results coming back days or even weeks later. With the addition of the rapid tests analyzed on site, the state determined that the clinic was functioning as a laboratory. It also challenged the accuracy of this type of test in diagnosing COVID-19.
The health department’s Division of Public and Behavioral Health “investigated and substantiated a complaint of the operation of an unlicensed laboratory,” according to a news release. “The laboratory was found to be collecting patient specimens and performing laboratory tests during an unannounced, onsite complaint investigation by the Division on Tuesday afternoon.”
Serology tests, which are blood tests that identify antibodies, are “not to be used for diagnostic purposes,” according to the news release.
A spokeswoman for the division did not respond to requests to explain why serology tests should not be used for this purpose.
“Any serological test at Sahara West Urgent Care & Wellness would not have a definitive result for the purposes of diagnosing COVID-19,” according to the new release. “Patients are urged to contact their health care provider to determine if further testing is required.”
Representatives of Sahara West stood by the accuracy of their test. They said they had unsuccessfully attempted to get guidance early on from the state regarding regulations and would work to meet any requirements..
Test accuracy
This week’s developments have left clinic patients confused.
Las Vegas resident Theresa Smith got the rapid test on Monday at Sahara West after she was sent home from work last week with a cough.
The test, she said, came back negative.
“But how am I to know?” she said. “What really is this test?”
The test, manufactured by Premier Biotech, is being used by researchers at Stanford University to study how many people are infected with, or recovering from, COVID-19, according to published reports. The test looks for two antibodies, one that develops in response to the initial exposure and another that develops later. The antibody that develops later may indicate that a person has some immunity to the disease.
No rapid blood tests have been approved by the U.S. Food and Drug Administration for COVID-19 testing, but because of the public health emergency the federal agency has allowed tests by various manufacturers to be distributed.
Stanford’s use of the test gave it credibility with Sahara West, said Mike Bradley, a researcher working with the clinic. The clinic performed a nasal swab test on all patients who had tested positive using the rapid test, as well as some patients who tested negative, to see if the results matched up. In a sampling of 50 patients, all the tests matched up, he said.
Bradley said that the test was intended to be read on the premises, not in a laboratory. But the state’s assessment was that the complexity of the text required the presence of a trained lab director, he said.
The clinic is working to comply with the state’s requests, and for the time being has halted all its COVID-19 testing, including the nasal-swab tests.
“We want to get those regulations and those hoops we need to jump through really quickly,” he said.
Expanded testing
Meanwhile, UNLV Medicine, the clinical arm of the UNLV School of Medicine, is expanding its drive-thru testing for COVID-19, which uses nasal swabs and delivers results typically within days.
The medical practice doesn’t plan to use rapid blood tests in its drive-thru testing in the near future, in part because of questions of quality and logistics.
“Where do they park?” asked Dr. Michael Gardner, UNLV School of Medicine’s vice dean of clinical affairs, in regards to patients waiting for results. “If you want a rapid test, you probably want a rapid answer.”
At this point, Gardner thinks that rapid tests are most needed in hospital settings to diagnose patients and for health care workers on the front lines of fighting the disease.
Antibody tests would be useful in assessing people’s risk of contracting COVID-19, he said.
“I‘d like to know if I have been exposed and have developed immunity,” he said. “A lot of us would like to know if we have immunity, if this is going to drag on.”
Antibody testing has been touted as a strategy both for combating the disease and for helping people get back to work.
The state is poised to launch its own testing for antibodies. In a briefing Wednesday with reporters, Mark Pandori, director of the Nevada State Public Health Laboratory, said antibody tests were to be shipped to the public lab as soon as next week, with testing ramping up in the coming weeks.
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.